Radhe Radhe, B.Pharm students! In this post, you will find the notes for your 1st semester Pharmaceutical Analysis subject . Here you will find notes, books, previous year questions (PYQs), and important question-answers related to your pharmacy course. You will also find GPAT updates here.

As you may have realized, pursuing a B.Pharm degree is not easy. Many people initially choose it as just one option, but later they discover how much studying is involved. Your B.Pharm course is divided into 8 semesters. In the first semester, you have to study a total of 6 subjects, of which 4 are compulsory. Those who passed their intermediate exams with Biology have to study Remedial Mathematics, and those who passed with Mathematics have to study Remedial Biology.
The link to download notes for all units is given below. If you want to download the PDF without reading the post, you can download the PDF by clicking on the link given below. You can also join our Telegram channel for future updates.
The Subjects in the 1st semester of B.Pharm are:
- Human Anatomy and Physiology- I
- Pharmaceutical Analysis
- Pharmaceutical Inorganic Chemistry
- Pharmaceutics I
- Communication Skills
- Remedial Mathematics
- Remedial Biology
Also Read:- Who is Hardik’s new girlfriend, Mahika Sharma?
B.Pharm 1st Semester Pharmaceutical Analysis Syllabus
Before downloading the notes, please take a look at the Pharmaceutical Analysis syllabus to understand what topics are covered.
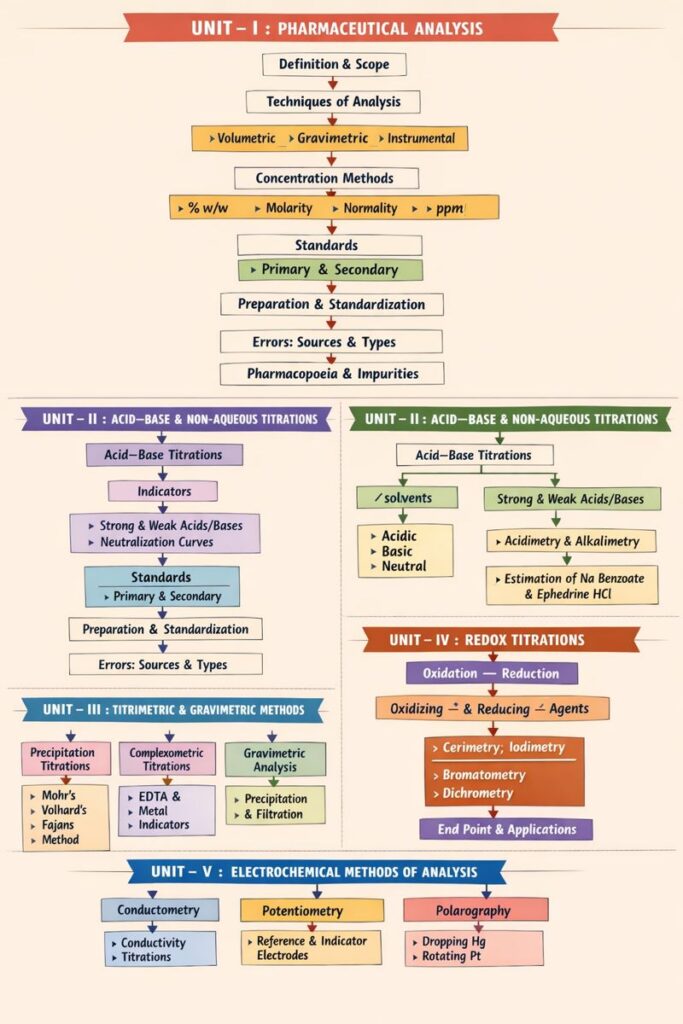
UNIT – I : Pharmaceutical Analysis
A. Pharmaceutical Analysis
- Definition and Scope of Pharmaceutical Analysis
- Techniques of Analysis
- Volumetric analysis
- Gravimetric analysis
- Instrumental analysis (overview)
- Methods of Expressing Concentration
- Percentage strength (% w/v, % w/w, % v/v)
- Molarity
- Normality
- Molality
- Parts per million (ppm)
- Standards
- Primary standards – definition, characteristics, examples
- Secondary standards – definition, examples
- Preparation and Standardization of Solutions
- Oxalic acid
- Sodium hydroxide
- Hydrochloric acid
- Sulphuric acid
- Sodium thiosulphate
- Potassium permanganate
- Ceric ammonium sulphate
B. Errors in Analysis
- Sources of errors
- Types of errors
- Systematic errors
- Random errors
- Gross errors
- Methods of minimizing errors
- Accuracy and precision
- Significant figures
C. Pharmacopoeia and Impurities
- Pharmacopoeia – definition and importance
- Sources of impurities in medicinal agents
- Limit tests – principle and applications
UNIT – II : Acid–Base and Non-Aqueous Titrations
A. Acid–Base Titrations
- Acid–base theories
- Indicators
- Theory of acid–base indicators
- Classification of acid–base titrations
- Theory of titrations involving:
- Strong acid vs strong base
- Weak acid vs strong base
- Strong acid vs weak base
- Very weak acids and bases
- Neutralization curves and their significance
B. Non-Aqueous Titrations
- Introduction and need for non-aqueous titration
- Solvents used in non-aqueous titrations
- Acidimetry and alkalimetry
- Estimation of:
- Sodium benzoate
- Ephedrine hydrochloride
UNIT – III : Precipitation, Complexometric, Gravimetric and Diazotization Titrations
A. Precipitation Titrations
- Principle
- Methods:
- Mohr’s method
- Volhard’s method
- Modified Volhard’s method
- Fajans method
- Estimation of sodium chloride
B. Complexometric Titrations
- Principle and classification
- Metal ion indicators
- Masking and demasking agents
- Estimation of:
- Magnesium sulphate
- Calcium gluconate
C. Gravimetric Analysis
- Principle and steps involved
- Purity of precipitate
- Co-precipitation
- Post-precipitation
- Estimation of barium sulphate
D. Diazotisation Titration
- Basic principles
- Methods
- Applications
UNIT – IV : Redox Titrations
A. Basic Concepts
- Oxidation and reduction
- Oxidizing and reducing agents
B. Types of Redox Titrations (Principles and Applications)
- Cerimetry
- Iodimetry
- Iodometry
- Bromatometry
- Dichrometry
- Titration using potassium iodate
UNIT – V : Electrochemical Methods of Analysis
A. Introduction to Electrochemical Methods
B. Conductometry
- Introduction
- Conductivity cell
- Conductometric titrations
- Applications
C. Potentiometry
- Electrochemical cell
- Reference electrodes:
- Standard hydrogen electrode
- Silver–silver chloride electrode
- Calomel electrode
- Indicator electrodes:
- Metal electrodes
- Glass electrode
- Methods for determining end point
- Applications
D. Polarography
- Principle
- Ilkovic equation
- Electrodes:
- Dropping mercury electrode
- Rotating platinum electrode
- Applications
B.Pharm 1st Semester Pharmaceutical Analysis Notes PDF
📘 Download B.Pharm – Pharmaceutical Analysis-I Notes (Unit-wise)
📥 Unit I – Pharmaceutical Analysis & Errors (PDF) 📥 Unit II – Acid-Base & Non-Aqueous Titrations (PDF) 📥 Unit III – Precipitation, Complexometric & Gravimetric Methods (PDF) 📥 Unit IV – Redox Titrations (PDF) 📥 Unit V – Electrochemical Methods of Analysis (PDF)📲 All Pharmaceutical Analysis notes are available on Telegram for fast & free access.

Also Read:- Human Anatomy And Physiology I Notes
B.Pharm 1st Semester Pharmaceutical Analysis Book PDF
Books are very important; you should definitely check out the books according to your syllabus. During your pharmacy studies, books from Nirali Prakashan and PV Sindhu are very helpful. You can download the PDFs from the link below.
- Pharmaceutical Analysis Nirali Prakashan Full Book PDF:- PDF
- Pharmaceutical Analysis PV Sindhu Full Book PDF:- PDF
Also Read:- B.Pharm 1st Semester Remedial Mathematics Notes
B.Pharm 1st Semester Notes
| Subject Name | Notes Link |
|---|---|
| Human Anatomy and Physiology- I Notes | View Notes |
| Pharmaceutical Analysis Notes | View Notes |
| Pharmaceutical Inorganic Chemistry Notes | View Notes |
| Pharmaceutics I Notes | View Notes |
| Communication Skills Notes | View Notes |
| Remedial Mathematics Notes | View Notes |
| Remedial Biology Notes | View Notes |
6 thoughts on “B.Pharm 1st Semester Pharmaceutical Analysis Notes”